News
Boston Analytical Rebrands as BA Sciences
Setting the Standard for Excellence in Global Life Sciences Analytical Testing Salem, NH – March 4, 2024 – Since its founding in 1987, Boston Analytical has been a trusted provider of high-quality analytical testing services to life science companies worldwide. With a commitment to excellence, compliance, and innovation, the company has established itself as a ... Boston Analytical Rebrands as BA Sciences
New State-of-the-Art Microbiology Laboratory Nears Completion for Grand Opening
BA is thrilled to announce the completion of our state-of-the-art microbiology laboratory, which is now in the final stages of preparation for its grand opening. Our dedicated team has been hard at work installing and validating cutting-edge support systems that will facilitate the highest level of laboratory research. From our RO/DI water purification system to ... New State-of-the-Art Microbiology Laboratory Nears Completion for Grand Opening
See you are these fall conferences!
ISPE Boston Area Chapter Product Show September 20 | Foxboro, MA Booth #W62 22nd Annual Contract Pharma September 21-22 | New Brunswick, NJ Technology Symposium October 2-3 | Holmdel, NJ Booth #609 2023 PDA Pharmaceutical Microbiology Conference October 2-4 | Washington, DC Booth #200 American Pharma Outsourcing Summit October 9-10 | Boston, MA Booth #19 ... See you are these fall conferences!
Summary of Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) Guidance for Industry
In August of 2023, the FDA Center for Drug Evaluation and Research (CDER) published a guidance document for evaluating the acceptable intake limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs). This guidance provides manufacturers and applicants of drugs with a tool to calculate a recommended acceptable intake (AI) limit for NDSRIs. Prior guidance by the FDA ... Summary of Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) Guidance for Industry
Director’s Corner: Jeff Heiser
Jeff Heiser is the Chief Scientific Officer (CSO) of Microbial and Advanced Therapeutic Platforms at BA Sciences. After graduating from the University of New Hampshire with a degree in Microbiology Research in 2007, he entered the industry working for Pfizer Biologics at their site in Andover, MA. There he worked on rapid Microbiological methods for ... Director’s Corner: Jeff Heiser
Exciting Updates on our Microbiology Buildout at 12 Manor Parkway!
BA Sciences is pleased to announce that we are in the final phase of our microbiology laboratory buildout at our 12 Manor facility. This new laboratory space more than doubles the current square footage of the microbiology laboratory, which will increase its efficiency and capacity. The equipment in the space will be fully qualified prior ... Exciting Updates on our Microbiology Buildout at 12 Manor Parkway!
Where We Have Been and Where We Are Going: A Look At 2022 and Our Growth in 2023
We had a pretty remarkable 2022! Take a look at what's in store for 2023!
Essential Experts: Chathurika Rathnayake, Ph. D.
Chathurika Rathnayake, Ph. D., is the Manager of Analytical Development at BA Sciences, Inc. in Salem, NH. She has been with BA Sciences since 2019, serving first as a Senior Scientist, then as Group Leader, and now in her current role. She oversees a group of talented Senior Scientists who perform analytical development, optimization, feasibility ... Essential Experts: Chathurika Rathnayake, Ph. D.
BA Sciences has first FDA inspection in three years – resulting in zero observations
SALEM, New Hampshire – BA Sciences, a leading provider of analytical and microbiological testing to the pharmaceutical, biopharmaceutical, and medical device industries is pleased to announce that their most recent FDA inspection resulted in no 483 observations. This was a general cGMP compliance audit focusing on veterinary and human drugs. Approximately 350 records and procedures ... BA Sciences has first FDA inspection in three years – resulting in zero observations
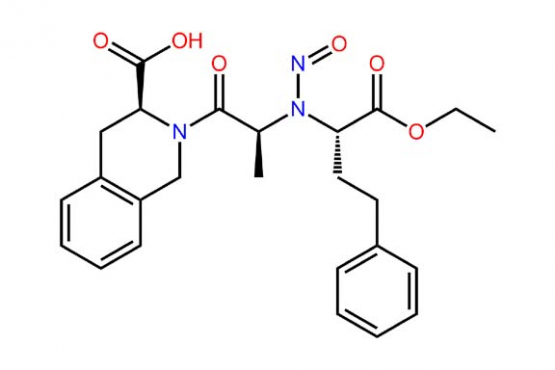
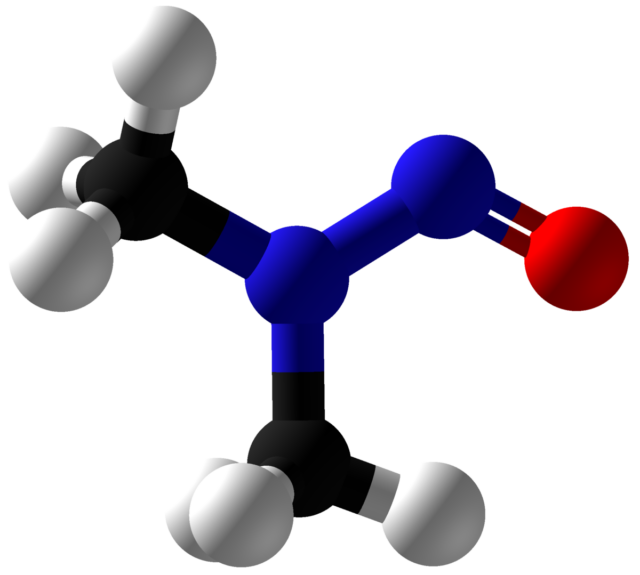
Nitrosamine Drug Substance-Related Impurity Testing
Ensuring that drug products do not contain nitrosamine compounds is vitally important given their toxicity. Nitrosamines can only form under certain conditions (luckily), however their high toxicity predicates the need to test for them at very low levels. BA Sciences provides nitrosamine testing in drug products and drug substances. We offer screening for analysis of ... Nitrosamine Drug Substance-Related Impurity Testing
Director’s Corner: Mark Guevarra
Mark comes to BA Sciences with 16+ years of experience working in the pharmaceutical industry. He currently is the Director of Analytical Development at BA Sciences. Previously Mark was a Senior Group Leader managing all analytical method validations/verifications, clients, and projects in the CRO space associated with Extractables & Leachables. He has direct knowledge in ... Director’s Corner: Mark Guevarra
Director’s Corner: Karina D. Allen-Ludwig, PhD
Karina has been with BA Sciences since 2017 and is the Director and Principal Scientist in the Biological Macromolecules Laboratory. In addition, Karina has had previous experience researching and developing methods in our Analytical Development Laboratory. She holds a PhD from Umass Lowell in Chemistry, with a focus on Biochemistry. Her research focused on the ... Director’s Corner: Karina D. Allen-Ludwig, PhD
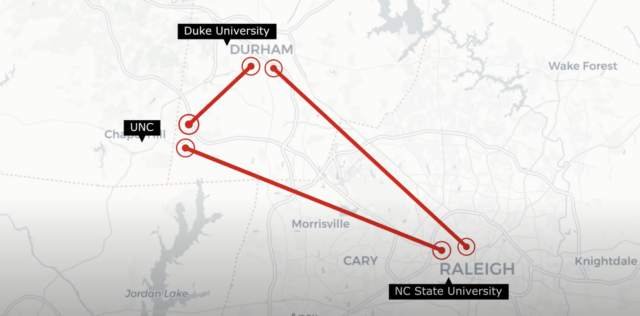
BA Sciences expands into Research Triangle Park Region (RTP)
Morrisville, North Carolina – BA Sciences, a cGMP compliant analytical laboratory headquartered in Salem, NH, is now opening their second location in Morrisville, NC. Services provided at this location will be sampling and testing services to support Environmental Monitoring, Monitoring of Critical Utilities including water systems and compressed gases. This location is the ideal area ... BA Sciences expands into Research Triangle Park Region (RTP)
Director’s Corner: Greg Slack, PhD
Greg is the Senior Director of a new area at BA Sciences, Scientific Research and Services. In this role, he draws on his 20 years experience in scientific management and 10 years as a bench scientist to simplify and streamline internal business processes, resulting in increased responsiveness to client requirements. Greg provides technical leadership in ... Director’s Corner: Greg Slack, PhD
Essential Experts: Shaoyuan(Jim) Zhang, Ph.D.
Dr. Zhang has been with BA Sciences since 2017 and works with the Extractables and Leachables (E&L) department as a senior scientist. He mainly focuses on Identification and quantification of non-volatile organic E&L compounds using LC-MS-TOF. Dr. Zhang also has a lot of experience in special case compounds such as Polyaromatic hydrocarbons (PAHs) and nitrosamines ... Essential Experts: Shaoyuan(Jim) Zhang, Ph.D.
E&L Case Study for Cream-Gel Topical Drug Product
Abstract: Topical creams and gels are used to treat dermal conditions, and also incorporate the active ingredient of the drug transdermally into the circulatory system through the skin. Topical products have many applications, however they present unique leachables concerns compared to traditional drug products. Topical creams and gels typically take the form of an oil-in-water ... E&L Case Study for Cream-Gel Topical Drug Product
Director’s Corner: Yvonne Nyavor, PhD
Yvonne has been with BA Sciences since 2021 and works with the Microbiology Department as the Principal Scientist for Microbiome. In Addition, Yvonne works with Business Development and Client Services, and collaborates with Analytical Development, Raw Materials/Chemistry and Large Molecules on Microbiome related projects. She has a PhD in Neuroscience from the University of Idaho ... Director’s Corner: Yvonne Nyavor, PhD
Essential Experts: Anthony Cannella, Ph.D.
Tony most recently graduated from SUNY University at Buffalo with a PhD in chemistry that focused on organometallic and inorganic synthesis and characterization via spectroscopic techniques. Also, he graduated with a BS in chemistry from the Rochester Institute of Technology (RIT) located in Rochester, NY. Tony has a lot of experience applying spectroscopic techniques like ... Essential Experts: Anthony Cannella, Ph.D.
GC-MS/LC-MS for the Analysis of Nitrosamines
Nitrosamines problem: Nitrosamines belong to a well-known group of carcinogens known as N-nitroso compounds because of their reactivity with DNA metabolizing into potent carcinogen diazonium ions. Nitrosamine formation lies in the oxidative release of the known nitrosating agents and subsequent nitrosamine formation by reaction with secondary and tertiary amines or dealkylated ammonium salts under acidic ... GC-MS/LC-MS for the Analysis of Nitrosamines
Director’s Corner: Mike Molloy
Mike has been with BA Sciences since 2012 and oversees the Biological Macromolecules Laboratory as the Technical Director. In Addition, Mike has had previous experience managing our Analytical Development and QC Chemistry Laboratories. He notably holds a master’s degree in biology from Umass Lowell and a bachelor’s in biology from UNH. One of the programs ... Director’s Corner: Mike Molloy
BA Sciences announces the appointment of Bob Coughlin to its Board of Directors as of February 8, 2021
Coughlin brings over 14 years of Biotechnology experience as the former President/CEO of MassBio SALEM, New Hampshire – BA Sciences, a leading provider of analytical and microbiological testing to the pharmaceutical industry, is delighted to announce the appointment of Bob Coughlin to its Board of Directors and Advisory Team. Coughlin brings a wealth of Biotechnology ... BA Sciences announces the appointment of Bob Coughlin to its Board of Directors as of February 8, 2021
Virtual Conference Series: Outsourced Pharma East Coast
During this unprecedented period, we at BA Sciences are aware of uncertainties you and your businesses may be facing and are here to help in any way possible with your analytical testing needs. Being sensitive to the fact that schedules, conferences and events have all been disrupted during this time we’re doing our best to ... Virtual Conference Series: Outsourced Pharma East Coast
Virtual Conference Series: BIOMEDevice
During this unprecedented period, we at BA Sciences are aware of uncertainties you and your businesses may be facing and are here to help in any way possible with your analytical testing needs. Being sensitive to the fact that schedules, conferences and events have all been disrupted during this time we’re doing our best to ... Virtual Conference Series: BIOMEDevice
Virtual Conference Series: Interphex
During this unprecedented period, we at BA Sciences are aware of uncertainties you and your businesses may be facing and are here to help in any way possible with your analytical testing needs. Being sensitive to the fact that schedules, conferences and events have all been disrupted during this time we’re doing our best to ... Virtual Conference Series: Interphex